Radoslav R. Adzic
Chemistry Department, Brookhaven National Laboratory, Upton, NY 11973, U.S.A.
Fuel cells, the devices that convert directly chemical energy of fuels into electrical energy, are considered to become a major source of clean energy. Particularly attractive is their automotive application that would produce enormous environmental benefits. A widespread application of fuel cells has been hampered by i) the cost of the catalysts (Pt) for fuel oxidation and oxygen reduction reactions, ii) their insufficient activity and iii) inadequate stability.
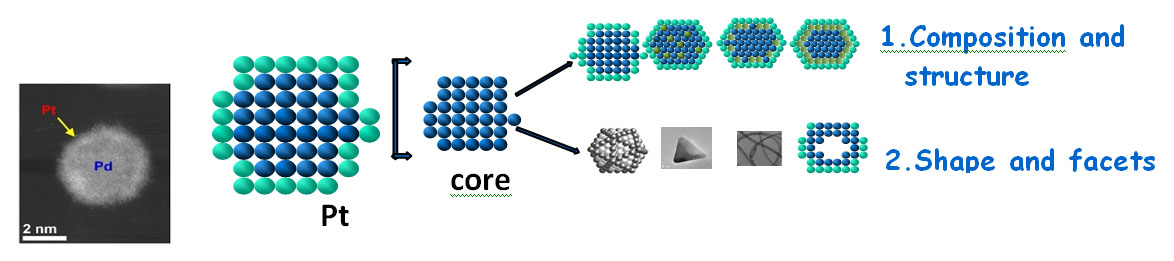
Although considerable advances in developing fuel cell catalysts were made over the last fifteen years, the amount of platinum used in electrocatalysts for the oxygen-reduction reaction (ORR) at fuel-cell cathodes remains large because of its insufficient catalytic activity and stability. In addressing those drawbacks, we developed Pt monolayer (PtML) electrocatalysts consisting of a single-layered shell of Pt atoms on cores of metal- or alloy- nanoparticles1,2. The origin of their high activity was identified by electrochemical- and surface-science techniques, x-ray absorption spectroscopy, and density functional theory calculations. The Pt monolayer electrocatalysts offer several uniquely attractive properties including an ultra-low Pt content, 100% Pt utilization and very high activity. High stability induced by supporting nanoparticle cores, self-healing property and fuel cell tests indicate that these electrocatalysts are ready for application and are established as a viable practical concept2,3. Further improving of these electrocatalysts can be achieved by changing the composition, shape, and size of cores to optimize the core-shell interaction, thus tuning their activities (Figure 1). The unique features of PtML electrocatalysts enable a wide selection of substrates to attain an electrocatalyst having low noble-metal content with enhanced catalytic activity and stability.
Several synthetic processes can be used to obtain the supporting cores, while the main method for placing a Pt monolayer on these core-shell nanoparticles involved the galvanic displacement of a Cu monolayer obtained by underpotential deposition by Pt. Other properties and further improvements of these catalysts will be discussed at the meeting.
Acknowledgements
This work is supported by U.S. Department of Energy, Divisions of Chemical and Material Sciences, under the Contract No. DE-AC02-98CH10886.
References
(1) Zhang, J.; Vukmirovic, M. B.; Xu, Y.; Mavrikakis, M.; Adzic, R. R. Angew. Chem. Int. Ed 2005, 44, 2132, https://doi.org/10.1002/anie.200462335
(2) Adzic, R. R.; Zhang, J.; Sasaki, K.; Vukmirovic, M. B.; Shao, M.; Wang, J. X.; Nilekar, U. A.; Mavrikakis, M.; Valerio, J. A.; Uribe, F. Topics in Catalysis 2007, 46, 249, https://doi.org/10.1007/s11244-007-9003-x
(3) Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R. R. Science 2007, 315, 220, https://doi.org/10.1126/science.1134569
Figure 1 SEM image of a Pt monolayer shell on a Pd core nanoparticle (left panel). Schematics of possible ways for tuneing Pt monolayer catalysts properties through core-shell interactions.